On Monday, June 6, another meeting of the Transparency Council.
The agenda includes:
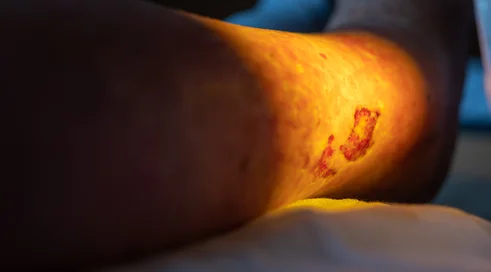
Preparation of a position on the evaluation of Dupixent (dupilumabum) under the drug program: "Treatment of patients with severe form of atopic dermatitis (ICD-10: L20).
Preparation of an opinion on the reimbursement of drugs containing the active substance posaconazolum in the following indications:
• financed under the pharmacy list: acute lymphoblastic leukemia in children up to 18 ...
Content locked
To gain access to the complete English section of the Medexpress.pl, kindly reach out to us at english@medexpress.pl.
If you already have an account, please log in